


ALPHOID DNA VARIATIONS AND NON-DISJUNCTION
IN DOWN’S SYNDROME: FLUORESCENCE IN SITU
HYBRIDIZATION AND CYTOGENETIC STUDIES
Vorsanova SG1,*, Yurov YB2, Beresheva AK1, Iourov IY2,
Monakhov VV2, Sharonin VO1, Demidova IA2, Kravets VS1
*Corresponding Author: Professor Svetlana G. Vorsanova, DSc., Director, Molecular-Cytogenetic Laboratory of Neuropsychiatric Diseases, Institute of Pediatrics and Children Surgery, Russian Ministry of Health, Taldom¬skaya str 2, 127 412 Moscow, Russia; Tel.: +7-095-484-19-48; Fax: +7-095-952-89-40; E-mail: y_yurov@yahoo. com
page: 81
|
|
RESULTS
Cytogenetic analysis provided us with an evaluation of the non-disjunction origin in 27 out of 36 families (75%). Non-disjunction of chromosome 21 was paternal in eight cases and maternal in 19 cases, corresponding to 29.6 and 70.4%, respectively (Table 1). Among the cases of maternal origin, 11 were of maternal meiosis I (MI), four were of maternal meiosis (MII), four were semi-informative maternal MI or MII. Among the cases of paternal origin, three were of paternal meiosis I (MI), two were of paternal meiosis (MII), three were semi-informative paternal MI or MII detected by cytogenetic analysis (Table 2). The meiosis stage was determined according to differences in morphological pecularities of chromosome 21. Examples of detection of parental origin and meiosis stage of non-disjunction are presented on Fig. 1.
The results of the evaluation of centromeric alphoid variants in chromosome 21, according to the method of Verma et al. [8] with minor modifications, are presented in Table 1. Sizes of the FISH signal of chromosome 21 were divided into five classes: very small (1); small (2); medium (3); large (4); and very large (5). These FISH variations were arbitrarily classified into five classes by comparison with the length of the short arm (p) of chromosome 18 [8]. We have analyzed 36 families with a DS child (36 probands, 36 mothers and 31 fathers). Negative FISH signal or hybridization signal absence was detected in only one mother’s chromosome 21 (#4 in Table 1). Chromosomes 21 with a very large FISH signal (class 5) corresponding to the length of the short arm (p) of chromosome 18 was not detected at all.
The analysis of the segregation of centromeric heteromorphic FISH variants in DS families allowed the determination of the origin of additional chromosome 21 in 20 of the 36 families (55.5%). According to the data obtained, paternal origin of chromosome 21 was found in six families and maternal origin in 14 families, corresponding to 30 and 70%, respectively. Among the maternal origin cases, one was of maternal MI, six were of maternal MII, seven were semi-informative maternal MI or MII. Among the paternal origin cases, four were of maternal MII and two were semi-informative paternal MI or MII. Paternal MI was not detected at all (Table 2). The mean value of the sizes of alphoid DNA heteromorphic variants was 2.2 for DS children, 2.2 for mothers and 2.25 for fathers (Table 1), i.e., these parameters were practically identical.
In order to compare the incidence of different classes of centromeric alphoid DNA classes (1, 2, 3, 4, 5) we have determined the frequencies (in %) of each class in the three groups analyzed: children with trisomy 21, their mothers and fathers (Fig. 2). The incidences of class 1 (very small) were 18, 22 and 19% for probands, mothers and fathers, respectively. The incidences of class 2 (small) were 42, 39 and 41% for probands, mothers and fathers, respectively. The incidences of class 3 (medium) were 34, 31 and 35% for probands, mothers and fathers, respectively. The incidences of class 4 (large) were 6, 7 and 5% for probands, mothers and fathers, respectively. Therefore, there is no significant difference in incidence of centromeric alphoid DNA between probands, mother and fathers.
The total efficiency in detection of parental origin of non-disjunction in the present study is 80.5% (in 29 families the origin of non-disjunction was detected by both techniques). The cytognetic analysis allowed us to determine the origin of an additional chromosome 21 in seven cases not detected by the molecular-cytogenetic technique (Table 2). However, the molecular-cytogenetic technique provided us the possibility of determining the chromosome 21origin in two additional cases not detected by the cytogenetic technique.
Table 1. Cytogenetic and molecular-cytogenetic analysis of the chromosome 21origin and FISH variants of alphoid DNA in chromosome 21.
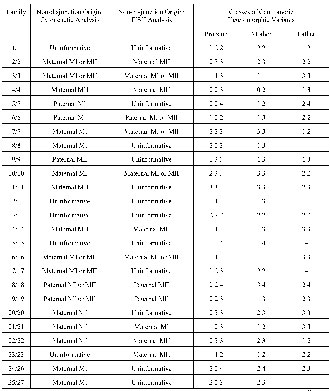
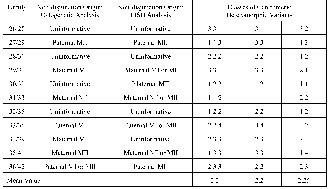
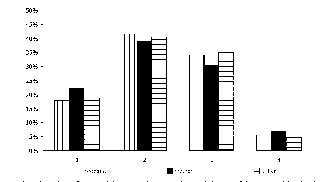
Figure 2. Incidence of centromeric heteromorphic variants (classes 1, 2, 3, and 4) of chromosome 21 (in %) probe in families with DS offspring revealed by FISH with alphoid DNA.
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

