


EVIDENCE FOR LARGE SCALE CHROMOSOMAL
VARIATIONS IN NEURONAL CELLS OF THE
FETAL HUMAN BRAIN
Yurov YB1,*, Vostrikov VS1, Monakhov VV1, Iourov IY1, Vorsanova SG2,*
*Corresponding Author: Professor Yuri B. Yurov and Professor Svetlana G. Vorsanova, Cytogenetic Labora¨tory; National Center of Mental Health, Russian Academy of Medical Sciences, Zagorodnoe shosse 2, Moscow 113 152, Russia; Tel.: +7-095-952-89-90; Fax: 7-095-952-89-40; E-mail: y_yurov@hotmail.com; y_yurov@ yahoo.com
page: 95
|
|
DISCUSSION
The present study indicates that the aneuploidy level was in the range of 0.2 to 15.6% for different chromosomes in the fetal brain cells. It means that the fetal neuronal cells to be characterized by increased frequency of non specific aneuploidy involved different chromosomes. Usually, fetal brain nuclei have one chromosome aberration, involving any individual chromosome, tested in interphase FISH experiments. Only limited numbers of nuclei had two or more aberrations, involving different chromosomes simultaneously. Approximately 95% neuronal cells could be considered as karyotypically normal, when we analyzed only one chromosome-specific DNA probe. However, mFISH analysis of several chromosomes in one FISH experiment, and application of a probe set for six different chromosomes, strongly indicated that the total number of chromosomally abnormal neuronal cells could be very high. For examples, we could estimate the total (cumulative) number of aberrant nuclei for six different chromosomes (1, 13/21, 18, X, Y) as 22%. We could propose that the number of aneuploidies in the fetal human brain cells, involving all 23 chromosome pairs, should be substantially higher. Therefore, our data strongly indicates that large-scale genomic (chromosomal) variations due to chromosomal complement instability in developing neuronal cells could take place and, therefore, this phenomenon can have a substantial effect on brain development.
There are no molecular-cytogenetic studies applying interphase FISH for the studies of the fetal human brain. Only the study of a model genetic object (mice) is available at the present time. Direct FISH and spectral karyotype analysis of neurons in the developing and adult nervous system of mouse embryonic cerebral cortical neuroblast have been performed [2]. These approaches identify more than 30% of neuroblasts to be aneuploid. Therefore, it is possible that genomes in developing and adult mammalian neurons can be different at the level of whole chromosomes.
There is only one indication, published in abstract form [6], that the fetal human brain could contain a large proportion of chromosomally abnormal cells. The results of the present study are in agreement with those data.
There are only limited numbers of molecular-cytogenetic studies, utilizing interphase FISH for the studies of the adult human brain [12,13]. The use of the one-color FISH technique to examine the chromosomal complement of interphase nuclei in the adult human brain have demonstrated that a significant fraction of the hyppocampal pyramidal and basal forebrain neurons in Alzheimerís disease have a fully of partially tetraploid chromosome complement. Yang et al. [12] propose that this imbalance in chromosome complement is the direct cause of neuronal loss in Alzheimerís disease. A molecular-cytogenetic study, utilizing mFISH of post-mortem brain of schizophrenic patients, has been recently performed [13]. A statistically significant level of aneuploidy (up to 4% of neurons) was detected in the postmortem brain (prefrontal cortex, Brodmannís area 10) of patients with schizophrenia. The result indicated that a low-level of aneuploidy (or chromosomal mosaicism) could be involved in the pathogenesis of schizophrenia. Therefore, neuropsychiatric diseases might have a special interest for extended molecular-cytogenetic analysis as they could be associated with chromosomal and gene mutations involving in regulation of neuro developmental processes in the brain. It was proposed recently that the central nervous system, both during development and in adulthood, has genetic mosaic features: an euploid population intermixed with a smaller but genetically diverse aneuploid population. Such mosaicism may have relevance to a variety of fields including stem cell biology, mammalian cloning, genomics, neurogenetics and neuropsychiatric diseases. At the organism level, these permanent genomic changes might contribute to physiological and behavioral variations among individuals not accounted for by classical genetics. Therefore, even extremely low percentages of mosaic forms of aneuploidies really exist in the fetal and adult human brain; the presence of abnormal neuronal cells could significantly affect normal brain development and functions.
Nevertheless, the actual amount of chromosomally abnormal neuronal cells in the human brain is unknown. Taking into account the data that large-scale genomic variations due to chromosomal complement instability in developing neuronal cells are present and could have substantial effect on normal brain development and functions, direct studies of chromosomal complements in the fetal and human brain should be continued.
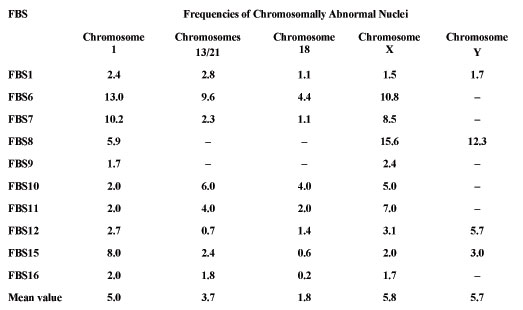
Table 1. The results of aneuploidy analysis in fetal brain samples (FBS) for chromosomes 1, 13/21, 18, X and Y (%).
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

