


ULTRASONOGRAPHIC MARKERS IN
CHROMOSOMAL ABNORMALITIES
Sifakis S*
*Corresponding Author: Stavros Sifakis, MD, 228 Oulaf Palme Street, 71410 Heraklion, Crete, Greece; Tel.: +302810392609; Fax: +302810212915; E-mail: sifakis@excite.com
page: 31
|
|
THE GENETIC SONOGRAM
Second trimester genetic sonography is a targeted examination of the fetus for the presence or absence of aneuploidy markers, the results of which can be used to adjust the a priori risk for DS [64]. The combination of multiple sonographic markers has been proved to be more sensitive than any single marker in the detection of fetal aneuploidy. By defining as abnormal any sonogram with one or more markers, many investigators have achieved detection rates of fetal DS greater than 80% [2,5,6,8,9,72-74].
In the USA, the second trimester genetic sonogram (15-23 weeks) is usually offered to pregnant women at increased risk for trisomy 21 due to advanced maternal age (at least 35 years old at delivery) or abnormal maternal serum biochemistry, or a combination of both [75]. In one study, the proportion of the above groups of the women undergoing genetic sonogram was 63, 27, and 10%, respectively [75].
Sonographic Scoring Systems for Aneuploidy Detection. A number of investigators have demonstrated that multiple sonographic markers can be instrumental in detecting fetuses with trisomy 21. Benacerraf et al. [8,76] have proposed a sonographic score for the detection of DS and other chromosomal abnormalities. They categorize the sonographic markers as “major” and “soft”, receiving a score of 2 and 1, respectively (Table 2). The “major” markers, even if isolated, should prompt consideration for karyotyping. The “soft” markers, are usually present in normal fetuses, and if isolated, do not necessarily lead to invasive testing. Benacerraf’s group have shown that if amniocentesis is reserved for fetuses scoring 2 or more, 73% of fetuses with trisomy 21 and 85% of fetuses with trisomy 18 can be identified, with a false-positive rate of 4% [8]. The same scoring system can be used to decrease the risk of trisomy 21 in patients at high-risk for an affected fetus, either because of advanced maternal age or an abnormal triple screen. For example, given a score 0 (a completely normal sonogram, with no markers identified), the risk for trisomy 21 can be diminished from 5/1,000 to 1.5/1,000 at age 35 years [8].
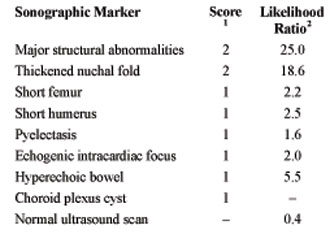
Nyberg et al. [33] developed the genetic sonogram via the age-adjusted ultrasound risk assessment for trisomy 21 based on maternal age and the likelihood ratios of a variety of sonographic markers (Table 1). The use of one or more markers could identify 68.3% of the fetuses with DS, with a 12.5% false-positive rate. It was also demonstrated that a normal sonogram could reduce the risk for trisomy 18 by a likelihood ratio of 0.4. Each likelihood ratio, multiplied by the maternal age-based risk, gives a revised final risk for the woman to have a fetus with trisomy 21.
Genetic Sonogram in High-Risk for Aneuploidy Pregnant Women. Bromley et al. [77] studied a group of women with advanced maternal age, applying the genetic sonogram to women who were candidates for amniocentesis but preferred non invasive testing. They were counseled that a score of 0 reduced by 50% the background risk for trisomy 21, while any positive score (>1) warranted amniocentesis. A sensitivity of 62.5% was found in the detection of DS, with a false-positive rate of 15%. These results among high-risk women are similar to those reported by Nyberg et al. [33] (68.3% detection rate, 12.5% false-positive rate). For high-risk women Vintzileos et al. [2] had a detection rate of 87% with a <20% false-positive rate.
Another large group of women at high-risk for aneuploidy is constituted by those women with an abnormal triple screen for aneuploidy (a combination of maternal age with the serum markers a-fetoprotein, estriol, b-chorionic gonadotropin). Many of these women choose to have an amniocentesis, but an increasingly high number are opting for the genetic sonogram before deciding whether or not to pursue amniocentesis [5]. This combination of triple testing results with sonographic markers allows a better estimation of the risk of the individual to have a fetus with aneuploidy. The results of many studies have shown that the frequency of sonographic markers of DS on this high-risk group of women was obviously high [78,79].
In the high-risk for aneuploidy group of women, due to advanced maternal age or to abnormal triple testing, the approach of undergoing a genetic sonogram before deciding whether or not to have an invasive procedure, is becoming widespread. Studies have shown that half of the women of this group choose this approach [5,78].
Genetic Sonogram: Indications and Limitations. The genetic sonogram is a detailed search for sonographic signs of aneuploidy, and can be used to identify fetuses at high risk for trisomies, as well as to decrease the risk for aneuploidy for a pregnancy when no sonographic markers are identified. Thus, second-trimester genetic sonography may be a reasonable alternative for pregnant women at increased risk for fetal trisomy 21 who wish to avoid amniocentesis. In experienced hands, this approach may result in a high detection rate of trisomy 21. Pregnant women should be advised that a normal genetic sonogram may theoretically reduce, but certainly cannot eliminate, the risk of trisomy 21.
Although recommending karyotyping when a sonographic marker is detected in a high-risk woman for aneuploidy is well accepted, the recommendation is less obvious when a single soft sonographic marker is detected in a pregnant woman at low-risk for fetal aneuploidy. Many investigators suggest that the combination of the genetic sonogram with maternal serum screening may be the best method of assessing the risk for aneuploidy in the second trimester of pregnancy [12].
Women at increased risk for aneuploidy, due to advanced maternal age or abnormal serum screening, can benefit from a genetic sonogram screening (for sonographic signs of aneuploidy) to adjust their baseline risk of an affected fetus. The use of genetic sonography in low-risk women requires further investigation [12]. The possible application of a genetic sonogram as a tool to the general or low-risk population is problematic for three reasons [75]. First, considerable expertise is required to rule out fetal structural malformations, especially subtle cardiac defects, and such expertise is not widely available. Second, due to the fact that multiple sonographic markers are evaluated, there is a 13-14% false-positive rate that is considered to be quite high for a screening test. Third, the genetic sonogram is time-consuming because of having to assess several ultrasound markers.
In the next few years, the methods that will compose the best and most sensitive combination for assessing an individual’s risk for aneuploidy will probably derive from a combination of the maternal age, the first and second trimester serum markers, the NT, the presence or absence of the nasal bone, as well as an early anatomical scanning at 13 weeks’ gestation, and the second trimester sonographic markers.
Table 2. The sonographic scoring1 system for the detection of trisomy 21 and 18 by Benacerraf et al. [8]. The ultrasound risk assessment for trisomy 21 based on the likelihood ratios2 of sonographic markers by Nyberg et al. [33].
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

