


ULTRASONOGRAPHIC MARKERS IN
CHROMOSOMAL ABNORMALITIES
Sifakis S*
*Corresponding Author: Stavros Sifakis, MD, 228 Oulaf Palme Street, 71410 Heraklion, Crete, Greece; Tel.: +302810392609; Fax: +302810212915; E-mail: sifakis@excite.com
page: 31
|
|
SONOGRAPHIC MARKERS FOR CHROMOSOMAL ABNORMALITIES
Nuchal Edema or Fold More Than 6 mm. It is found in about 0.1-0.5% of fetuses and it may be of no pathological significance [1,12]. It is, however, associated with chromosomal defects, cardiac anomalies, infection or genetic syndromes. The nuchal fold is measured from the outer edge of the occipital bone to the skin edge in a modified transverse view of the fetal head, which includes the brain stem, cerebellum, and the occipital bone. The measurement is considered abnormal if it is 6 mm or greater [8]. The nuchal fold measurement should only be used between 15 and 20 weeks, when the normal skin thickness is relatively constant [19].
Using a threshold of 6 mm, or greater, as an abnormal measurement, 40% of the fetuses with trisomy 21 were identified with a thickened nuchal fold [20]. In addition, many investigators were able to identify 40-75% of fetuses with DS, with a false-positive rate of <2% at between 15 and 20 weeks’ gestation [21-23]. For isolated nuchal edema, the risk for trisomy 21 may be 10-times the background risk [1]. Some investigators also support the hypothesis that a normal nuchal fold in a high-risk pregnant woman, due to advanced age or abnormal triple screen, may decrease the risk of aneuploidy [24].
Nuchal thickening in fetuses with trisomy 21 is thought to represent some form of lymphatic abnormality, similar to cystic hygromata. This finding may also be related to the abnormal NT of fetuses in the first trimester. It has certainly been shown that nuchal translucency is a very strong and much more sensitive marker for the detection of DS than the second trimester measurement of the nuchal fold. The measurement of fetal NT thickness at the 11-14-week scan combined with maternal age, can identify about 75% of pregnancies with trisomy 21. Moreover, combined with maternal serum markers of the first trimester (free-b-hCG, and pregnancy-associated plasma protein-A), the detection rate of trisomy 21 is about 90%. Increased NT can also identify a high proportion of other chromosomal abnormalities and is associated with major defects of the heart and great arteries, and a wide range of skeletal dysplasias and genetic syndromes [25].
A thickened nuchal fold is also associated with euploid syndromes, such as the Noonan syndrome, as well as with heart defects. Therefore, fetuses with a thickened nuchal fold should undergo a karyotype, as well as a fetal echocardiogram and a complete anatomical scan to rule out other malformations. A nuchal fold, representing the second-trimester form of NT, has been accepted by the majority of the investigators as the single most sensitive and specific marker for the detection of DS in the second trimester [12].
Hyperechogenic or Hyperechoic Bowel. The bowel is characterized as echogenic, when its echogenicity is similar to that of iliac bones. Hyperechogenic bowel is found in about 0.5-0.6% of fetuses [1,12] and is usually of no pathological significance. Initially, this finding was considered to be either a normal variant in second trimester fetuses [26], or a consequence of intra-amniotic bleeding, which probably is the most common cause [1]. More recently, some pathological conditions such as trisomy 21, cystic fibrosis, severe early-onset fetal growth retardation, cytomegalovirus infection and perinatal death, have been associated with second trimester hyperechogenic bowel [27-32].
The risk for aneuploidy in fetuses with hyperechogenic bowel ranges from 12.5 to 21.8%, with the majority having trisomy 21 [27,29-32]. However, the risk of DS in fetuses with isolated hyperechoic bowel in the general fetal population is 1.4%; 12.5% of the karyotyped fetuses with DS present this finding [31]. Thus, the relative risk for trisomy 21 in fetuses with isolated hyperechogenic bowel is 5.5 to 7 [1,33]. The correlation with abnormality is as high as the greatest echogenicity, equal to that of bone. This is the most accepted criterion [27], taking into account the subjective nature of this sonographic marker [33]. In any case, the identification of this marker should be accompanied by genetic counseling. If other sonographic markers are present, an amniocentesis for fetal karyotyping should be offered to the parents. Further clinical and laboratory investigation to rule out cystic fibrosis is also suggested.
Short Femur and Short Humerus. Fetuses with trisomy 21 have slightly shorter long bones (namely femur and humerus) than do normal fetuses. Between 50 and 68% of the second trimester fetuses with trisomy 21 present with shorter femur bone length [34]. Short femur is found four-times as often in trisomy 21 fetuses compared to normal fetuses [1]. Autopsy specimens of fetuses with trisomy 21 showed that the humerus was the most commonly affected bone length [35]. Benacerraf et al. [36] showed sonographically, based on biparietal diameter measurements, that 50% of fetuses with trisomy 21 had shorter humerus lengths than expected. Many investigators suggest that the shorter femora and humeri bones of the fetuses with DS is a useful finding in the sonographic detection of fetuses with DS, and therefore, a valuable marker in the genetic sonogram [34,37-39]. Moreover, it has been reported that a relative risk of 2.2 for femur and 2.5 for humerus length abnormalities can be multiplied by the age-based risk, or triple test risk, to create a modified risk of the fetus for trisomy 21 [1,33]. The diagnoses of short femur and short humerus are based on the criteria according to a given biparietal diameter (BPD) measurement [40], or to established nomograms [41]. Short long bones are rarely a sign of dwarfism.
A number of investigators, however, doubt the clinical usefulness of the fetal bone length estimation in DS detection [40,42-45]. One reason is that the variance of the length is not consistent enough to be clinically useful. Another reason is that the different somatic characteristics of the various populations prevent the evaluation of long bone lengths as a universal and accurate sonographic marker. Therefore, there is some evidence that the isolated short femur may not be more common in trisomic than normal fetuses. Moreover, if the femur is below the 5th centile and all other measurements are normal, the baby is likely to be normal but rather short [1].
What is currently believed is not the isolated measurement, but the combination of long bones biometry with the nuchal fold information, or with a multiple-markers setting, that may increase the detection rate of fetuses with DS in the second trimester [37]. Vintzileos et al. [2] considered the short humerus in common with cardiac structural anomalies and the abnormal nuchal fold thickening as the most important ultrasound markers for the detection of trisomy 21. The same investigator has further shown that the risk of fetal aneuploidy may be decreased in older pregnant women when the long bones are sonographically demonstrated to be normal or longer than the expected size [46].
Echogenic Foci in the Heart. Sonographically, this is a bright area (as bone) most often in the left ventricle, and occasionally bilateral or right-sided and located just below the mitral or triscupid valves. This echogenic intracardiac focus represents microcalcification in the papillary muscle or chordae tendinae of the fetal heart in the second trimester [17,47]. It is found in about 4-10% of pregnancies [1,48,49]. Echogenic foci, probably representing a normal variant [50,51], are usually of no pathological significance. Many investigators have shown an association between echogenic foci and aneuploidy [16,47,49,52-54]. Roberts and Genest [17] reported, after pathological examination, that calcification of the papillary muscle had 39% of the abortuses with trisomy 13, 16% of those with trisomy 21, and 2% of normal abortuses. Taking into consideration that echogenic foci are present in less than 20% of aneuploidy fetuses, as well as in 5-10% of normal fetuses, this sonographic marker cannot be characterized sensitive or specific. The relative risk for trisomy 21, after an isolated recognition of this marker in the sonogram, is two- or three-times that of a priori risk [1,33], and the need of invasive testing remains controversial. However, doubling the age-based risk may be important for pregnant women of 32-34 years old, but in a younger woman with a normal triple screen, the presence of an echogenus focus in the heart should not result in a final risk indicating an amniocentesis.
Choroid Plexus Cysts. These are found in about 1-2% of pregnancies and they are usually of no pathological significance [1]. When other defects are present, there is a high risk of chromosomal defects, usually trisomy 18 but occasionally trisomy 21 [55]. Choroid plexus cysts present in one-third of the fetuses with trisomy 18 [12]. On the other hand, there is no difference in the incidence of choroid plexus cysts in fetuses with and without trisomy 21 [56]. There is still controversy regarding whether or not to recommend amniocentesis after the sonographic recognition of isolated choroids plexus cysts [55-57]. Benacerraf et al. [14] calculated that the chance for a fetus with isolated choroids plexus cyst to have trisomy 18 is 1/477, while in a meta-analysis [57], this risk was also found to be low (1/374). For isolated choroid plexus cysts, the risk for trisomy 18 is 1.5-times the background risk [1]. On the other hand, when malformations are seen on the prenatal sonogram in the presence of choroids plexus cysts, the risk for aneuploidy is high, and karyotyping should be recommended.
Mild Hydronephrosis or Pyelectasis. Mild hydronephrosis is seen prenatally in 17-25% of the fetuses with trisomy 21 compared with 2-3% of normal fetuses [58-59]. The vast majority of the second trimester fetuses displaying a mild-to-moderate pyelectasis will not have any clinical consequence in the renal function after birth [58]. Although an association has been established between fetal renal pyelectasis and fetal DS, this sonographic marker is not considered sufficiently sensitive and specific to be used as an isolated finding for the DS antenatal detection [60]. Moreover, the isolated pyelectasis has been found to have a relative risk for trisomy 21 only 1.5 times the background risk [1,33]. The most used criterion for the definition of mild hydronephrosis or pyelectasis is the measurement of anteroposterior diameter of the fetal renal pelvis (measurements equal or greater than 4.0, 5.0 or 7.0 mm at 16-20, 20-30, and 30-40 weeks’ gestation, respectively) [58]. The current viewpoint for mild hydronephrosis is that only when other abnormalities are present, or the patient is at high risk for aneuploidy, should amniocentesis be considered.
The Iliac Angle. There is a wider lateral flare of the iliac bones in individuals with trisomy 21. This can be demonstrated in the second trimester in a transverse image of the fetal pelvis, in which the angle formed between the iliac crests (iliac angle) is measured [61-62]. The mean iliac angle in fetuses with trisomy 21 is 79° ± 19°, which is significantly different from the 67°±14° in normal fetuses [62]. However, because of the highly variable results of many investigators, as well as the wide differences in angles formed by the iliac bones at various levels, the measurement of the iliac angle has not so far been incorporated with other markers in the genetic sonogram [12].
Structural Anomalies. Under this heading are included severe structural anomalies of the face (flat facies with maxillary hypoplasia, macroglossia, brachycephaly), the hands (hypoplasia of the middle phalanx of the fifth digit, clinodactyly) and the heart. Many authors also include abnormalities such as duodenal atresia, ventriculomegaly, hydrops, and the clubbed foot [1,12].
Structural Anomalies of the Heart. Congenital heart disease is the most common anomaly in infants with DS, present at a rate of 40-50%. The most common defects are atrial and ventricular septal defects (45% atrioventricular septal defects-AVSD, 35% ventriculoseptal defects, 8% isolated secundum atrial septal defects). Other defects are persistent patent ductus arteriosus (7%), and isolated tetralogy of Fallot (4%) [63]. These defects can be missed even by experienced sonographers. It is estimated that in approximately 7% of the women who undergo a genetic sonogram, the fetal heart or the fetal face is not adequately visualized [64]. Perhaps the use of color flow imaging and Doppler ultrasonography will improve their identification.
Hypoplasia of the Middle Phalanx of the Fifth Digit, and Clinodactyly. The difficult measurement that leads to a subjective evaluation, as well as the high false-positive rate (about 18%), makes the incorporation of these markers problematic in the sonographic screening tools for aneuploidy [65].
Other Sonographic Signs. In the last few years, some additional characteristics of fetuses with trisomy 21 have received some attention, and have been investigated as candidate sonographic signs for antenatal detection. They are rather subtle features, and have not yet been proved to hold enough specificity and sensitivity to be used in genetic sonogram screening for aneuploidy. In addition, there are technical difficulties to many of these features for accurate evaluation.
Fetuses with chromosomal abnormalities, especially with DS, may have short ears and this can be detected in second trimester ultrasonography [66]. The measurement is difficult, and there is a large overlap between normal and abnormal fetuses as well [67]. Second trimester fetuses with trisomy 21 have slightly shorter frontal lobes [68], however, there is so much overlapping between normal and abnormal measurements that do not allow for using this marker in genetic sonograms (69). The wide space between the first and the second toes, also called “sandal gap foot”, is the separation of the great toe, usually determined by subjective evaluation [70]. Most investigators do not include this feature to fetuses with DS in the genetic sonogram. Recently, there was evidence that delayed fusion of the amnion and chorion is associated with an increased risk of DS [71]. This membrane abnormality is exceedingly rare.
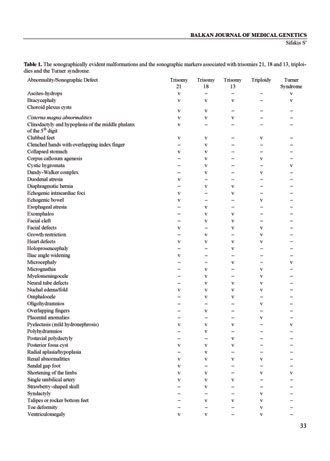
Table 1. The sonographically evident malformations and the sonographic markers associated with trisomies 21, 18 and 13, triploidies and the Turner syndrome.
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

