


ORGANOCHLORIDE PESTICIDES IN MACEDONIAN GIRLS
WITH PREMATURE SEXUAL DEVELOPMENT
Krstevska-Konstantinova M1,*, Kocova M1, Charlier C2, Bourguignon JP3
*Corresponding Author: Dr. Marina Krstevska Konstantinova, Pediatric Clinic, Department of Endocrinology and Genetics, Medical Faculty, Vodnjanska 17, 1000 Skopje, Republic of Macedonia; Tel.: +389-2-314-7474; Fax: +389-2-3225-809; E-mail: marina@lancom.com.mk
page: 43
|
|
DISCUSSION
Our previous study [3], showed that foreign adopted children and non adopted girls with precocious puberty tested for eight pesticides, detected p,pí-DDE, which is derived from the organochloride pesticide DDT, while Belgian girls with idiopathic or organic precocious puberty showed undetectable concentrations. A possible relationship between transient exposure to endocrine disruptors and sexual precocity was suggested [3]. This hypothesis was further investigated in our country, where we evaluated 56 girls with PSD for the presence of p,pí-DDE compared to a control group of 24 healthy girls. We expected to obtain results that would show higher pesticides levels in the girls with PSD, but our results did not confirm our hypothesis. We were surprised to find increased levels of lindane in both groups of girls in our population, which was not registered in the Belgian study. The levels of p, p'-DDE in our two groups of girls were similar to those in the adopted and non adopted foreign girls in Belgium. Two hypotheses were tested for the appearance of PSD in relation to xenoestrogens. One concentrated on the mechanism through which xenoestrogens, having estrogenic effect, weakly stimulate the estrogen-sensitive tissues including the hypothalamus, and enhance its maturation with the consequence of PSD. The weak stimulation of estrogen-sensitive tissues may occur peripherally, as seen in the epidemic of PSD, possibly influenced by xenoestrogens [12]. The stimulation of estrogen-sensitive tissues may also occur centrally. The other hypothesis suggests that an environment which is pesticide-free, while moving from an environment where they are present, may trigger the appearance of PSD. In the environment where the xenoestrogens are present, a negative gonadotropin feed-back inhibition exists. It is a well known central effect of estrogens in pre pubertal children [17,18].
The DDT isomers o,pí-DDT, o,pí-DDE and p,pí-DDT exhibit weak estrogenic activity in many systems, for example, the rat uterus [19,20]. Some estrogenic and anti-androgenic effects of p,pí-DDE have been reported in salamanders [21]. The results of Charlier [22], suggest that environmental exposure to p,pí-DDE, HCB, or PCBs may contribute to the pathogenesis of breast cancer and male infertility, and that deleterious effects are probably restricted to intra-uterine life. Diethylstilbestrol (DES) and o,pí-DDT given to pubertal rats act as estrogenic morphogens; i.e., they increase cell proliferation, which promotes maturation of the undifferentiated terminal end buds to more differentiated lobular terminal ductal structures [23]. Pathological variations in the timing of puberty may provide unique information about the interactions of environmental conditions or of genetic susceptibility with the hypothalamic mechanism that controls the onset of sexual maturation, as shown by examples of precocious puberty following exposure to endocrine disruptors or due to hypothalamic hamartoma [24].
Other substances also affect the timing of puberty. Early puberty was more common in daughters of mothers exposed to polybrominated biphenyl or PBB while breast-feeding their daughters. Menarche occurred in these girls with a mean of 11.6 years, compared to 12.7 years in the normal population [8]. In a study of 594 girls, it was seen that girls who had the highest level of PCB reached puberty earlier than their peers [25], while a study of 41 girls with premature thelarche showed that 68% had increased levels of phthalates, present in plastics, in comparison to a healthy control group [26].
Delayed puberty has also been reported after exposure to endocrine disruptors. A study of 3,000 girls from different ethnic groups in the USA found that increased concentrations of copper in the environment may result in delayed growth and puberty in girls [7]. The effect of polychlorinated aromatic hydrocarbonates (PCAH) on sexual maturation among 200 urban adolescents was evaluated and compared in rural adolescents; only those boys or girls who had elevated concentrations of the chemical experienced delayed puberty [27].
The use of DDT has been banned in West European countries and the USA for almost four decades [28]. A proposal has been presented to the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) to develop a minimum list of pesticides which are not hazardous for human health [29]. In the Republic of Macedonia, the law on Plant Protection banned the application of Persistent Organic Pollutants (POPs) from 1982. Six of the nine POP pesticides are subject to the "Rotterdam Convention on the Prior Informed Consent (PIC) Procedure for Certain Hazardous Chemicals and Pesticides in International Trade" in which the Republic of Macedonia was included in 2001. However, they remain in limited use, legally and illegally. The last application of DDT as an insecticide in the Republic of Macedonia was recorded in 1976 for plant protection of the forests [30].
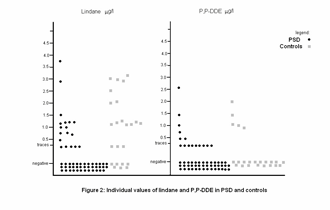
Figure 2. Individual values of lindane and p,pí-DDE in PSD girls and controls.
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

