


COMPARISON OF K-RAS MUTATIONS IN LUNG TUMOR
AND TUMOR-ADJACENT HISTOLOGICALLY-NORMAL
LUNG TISSUES OF PATIENTS WITH LUNG CANCER
Keohavong P1,5, Mady HH2,6, Gao WM1, Siegfried JM3,5, Luketich JD4,5,
Melhem MF2,6
*Corresponding Author: Dr. Phouthone Keohavong, Department of Environmental and Occupational Health, University of Pittsburgh, 3343 Forbes Avenue, Pittsburgh, PA 15260, USA; Tel: +412-383-2087; Fax: +412-383-2123; E-mail: pho1@pitt.edu
page: 3
|
|
RESULTS
Studies of K-ras mutations in lung tissues. In order to evaluate the role of K-ras mutations in pulmonary carcinogenesis and establish its significance as an early detection biomarker, one needs sufficiently sensitive and accurate methods that allow analysis of several samples simultaneously and detection of potential multiple mutations in each sample. Several molecular approaches have been applied to enhance the detection of point mutations in cultured cell, tissue, or sputum samples, including mismatch amplification mutation assay [25], polymerase chain reaction (PCR) and restriction length polymorphism [19,26-28], enriched-PCR [29-31], PCR-based cloning and specific probe hybridization [32], and ligase chain reaction [33]. Another specific and sensitive method has been developed for K-ras mutation detection by improvement upon existing methodology. Through a combination of PCR, mutant allele enrichment (MAE), nested amplification, and denaturing gradient gel electrophoresis (DGGE), a sensitivity of detection of one mutated cell in 104 to 105 normal cells can be attained. This method has been applied to analyze samples microdissected from formalin-fixed and paraffin-embedded sections of lung tissue obtained from patients with lung cancer [34].
The use of formalin-fixed and paraffin-embedded tissues enabled an accurate morphologic analysis and a precise topographic sampling of the tissue sections. Tissues taken outside the tumor area are carefully reviewed histologically to insure of the homogeneity of the cells sampled. Paraffin blocks of lung parenchyma adjacent to and those distant from tumor are topographically sampled in a similar manner. Control tissues, both negative and positive for K-ras mutations, are processed at the same time. After microdissection, the tissue sections are stained with hematoxin-eosin and compared to the original histologic sections to confirm the accuracy of the target selection. We had initially used this approach of tissue microdissection in combination with a sensitive method to analyze and compare K-ras mutations in lung tumor and matched tumor-adjacent histologically-normal tissues in pulmonary lectomy specimens from 38 patients with adenocarcinoma.
Histologically normal tissues taken from outside K-ras mutated lung tumors contained a low fraction of the mutational genotype identical to those found in the corresponding tumor in four of eight K-ras mutation-positive lung cancer patients. However, this original approach analyzed tissue samples that contained several hundreds cells each. There were some concerns that the low fraction mutations detected in the histologically normal tissues might have originated from contamination with a few undetectable tumor cells or mutated DNA diffusing from necrotic tumor cells in surrounding tissues, in spite of the necessary precautions taken to avoid such a possibility [34]. To test this possibility, a more accurate method has been applied to histologically review and precisely sample a few cells of interest in paraffin-embedded lung tissue sections for mutation analysis.
Analysis of K-ras mutations in cells taken by laser capture micro-dissection. The use of a laser capture allowed an accurate morphologic analysis and precise sampling of cells from interested areas of paraffin-embedded tissue sections. Histologically normal cells were individually taken by laser capture from the tissue area located between 1.5 and 5.0 cm from the edge of the tumor area. The approximately 100 cells captured on each Cap were again carefully reviewed histologically. All appeared homogeneously normal-looking and there were no tumor cells present. Paraffin-embedded tissue blocks representative of tumor tissue were topographically sampled in a similar manner. Figure 1 shows a representative example of a target-site sampling from a case of lung adenocarcinoma. The cells captured from the tumor tissue all appeared malignant histologically. After microdissection, each captured cell sample was compared to the matched tissue section, prior to and after micro-dissection, to confirm the accuracy of the target selection (see Figure 1A-C).
Figure 2 shows an example of molecular analysis by DGGE of K-ras mutants in laser captured-cell samples for one case of lung adenocarcinoma. Mutant alleles that separated from the wild type allele in the gel were isolated and further analyzed by sequencing. Mutation analysis of malignant cells sampled at multiple sites showed that the mutation identified was homogeneously distributed throughout the tumor, suggesting that these mutations might occur before tumor progression. A similar analysis of tumor-surrounding histologically-normal tissues showed that the identical mutation found in the tumor was also present in the matched histologically normal tissues. A summary of mutations identified in tumor and tumor-surrounding normal-appearing tissues for eight cases of lung cancer patients are shown in Table 1. Three cases (patients 2, 4, and 6) each contained an identical codon 12 mutation (GGT®TTT, GGT®GTT, and GGT®TGT, respectively) in both the tumor and normal-appearing tissues. In addition, further analysis showed that case 4 also contained a second codon 12 mutation (a GGT®AGT) in a site of the normal-appearing tissue. Case 1 contained two silent mutations in codons 9 (GTT®ATT) and 11 (GCT®ACT) in normal-appearing tissue, and two mutations (GGT®GAT and AGT) in codon 12 in tumor tissue.
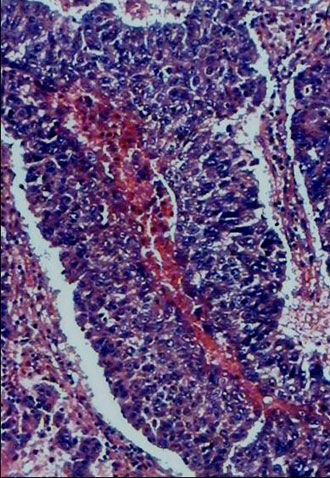
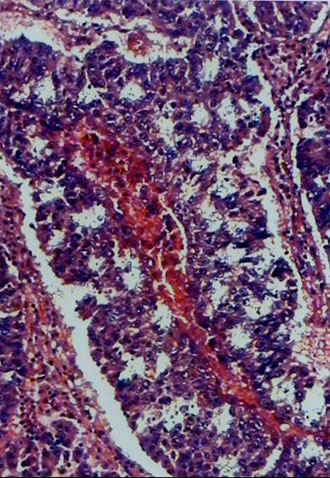
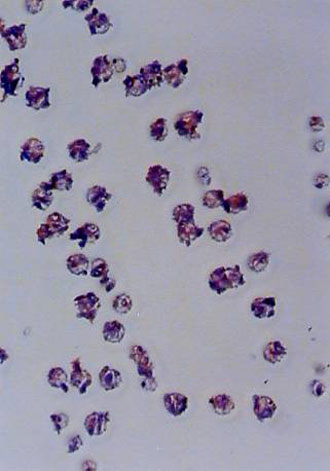
Fig. 1. An example of tissue site sampling from formalin-fixed and paraffin-embedded tissue sections from a case of lung adenocarcinoma, using a laser capture micro-dissection. The figure shows a pair of pre- (A) and post- (B) topographic tissue sections of lung tumor. Areas from histologically-normal tissues distant from the tumor were also sampled, but are not shown in the figure. Part C shows a sample of cells “laser-captured” on a Cap before treatment with proteinase K and used for molecular analysis.
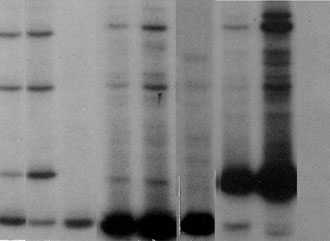
Fig. 2. DGGE analysis of K-ras mutations. K-ras gene exon 1 was amplified from cellular DNA isolated from each cell sample micro-dissected from histologically normal and tumor areas of selected lung tissue sections as shown in Fig. 1. The amplified DNA was then analyzed by DGGE to separate any mutant allele, that may be present in each DNA sample, from the wild type allele. The figure shows sequence patterns of K-ras mutant alleles in two malignant cell samples (T1 and T2) and in three matched histologically normal cell samples (N1, N2, and N3) taken from different areas of the tumor and tumor-adjacent normal tissues, respectively. Each lane of the gel shows the wild type K-ras codon 12 allele as a homoduplex fragment (Wt) and the mutant alleles separated from the wild type allele as three bands corresponding to a mutant homoduplex fragment (Hom.), and the two respective mutant/wild type heteroduplex fragments (Het.1 and Het.2). Lane C shows the sequence patterns of a known K-ras mutation-negative control lung tissue sample. Lanes n1, n2, and n3 represented the same DNA as in samples N1, N2, and N3, respectively, that had been subjected to mutant allele enrichment prior to analysis by DGGE to further improve the sensitivity of mutant allele detection.
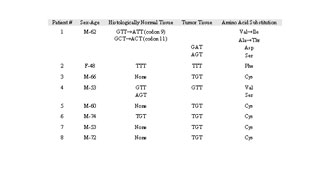
Table 1. Summary of K-ras mutations in lung adenocarcinoma
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

