


PRE-IMPLANTATION GENETIC DIAGNOSIS
USING POLAR BODIES
Tomi D1,*, Voigt R1, Eckhold J1, Hinrichs F1, Griesinger G2,
Shulze-Mosgau A2, Schöpper B2, Al-Hasani S2, Diedrich K2, Schwinger E1
*Corresponding Author: Dr. Diana Tomi, Institute of Human Genetics, Medical University of Lübeck, RatzeČburger Allee 160, 23538 Lübeck, Germany; Tel.: +49(0)451-500-2621; Fax: +49(0)451-500-4187; E-mail: dianatomi@hotmail.com
page: 17
|
|
MATERIALS AND METHODS
In all cases, investigations on PBs were approved by the institutional research ethics committee. Up to now, molecular genetic diagnosis for CF using PBs has been applied in two couples. The first couple (couple A) already has two affected sons (Fig. 1). They carry the DF508 mutation inherited from the mother and an unknown mutation inherited from the father. Figure 1 shows the pedigree of the family with the DF508 mutation and the linked polymorphic markers to that mutation. Affected and unaffected alleles are coloured red and green. Couple B (Fig. 2) has an affected son who is a compound heterozygote for the DF508 and G551D mutations. They have had three pregnancy terminations after prenatal diagnosis because all three cases revealed affected fetuses in. The DF508 mutation was inherited by the mother. Both couples underwent detailed genetic and gynecological counseling prior to the investigations.
First PBs were obtained after intracytoplasmatic sperm injection (ICSI) by laser assisted biopsy [8]. The biopsy of the second PBs was only performed in couple B followed by cryopreservation of the corresponding oocytes. Due to the time consuming procedure it was not possible to obtain the results from second PBs in the same cycle. Polar bodies were transferred into 0.5 mL micro-centrifugation tubes containing 5 mL of sterile distilled water overlaid with 50 mL mineral oil (Perkin Elmer, Foster City, CA, USA). Cell lysis was reached by adding 5 mL lysis solution (containing 20 mM TRIS-HCl, pH 9.0, 100 mM KCl, 3 mMgCl2, 0.2% Triton X-100, 0.4 mg/mL gelatine, 0.5 mg/mL Proteinase-K). The tubes were incubated at 55░C for 60 min. followed by inactivation of Proteinase-K at 95░C for 15 min. [9]. Multiplex PCR was performed with primers for the DF508 mutation region and informative linked polymorphic markers for each couple. After initial denaturation at 94░C for 5 min., the DNA was amplified for 25 cycles at 94░C, 55░C and 72░C for 30 sec., followed by a final extension of 10 min. at 72░C. Nested PCR for the mutation region and each polymorphic marker was performed at specific annealing temperatures with fluorescent labeled primers. The PCR products were resolved by 6% polyacrylamide gel electrophoresis (PAGE) on a Li-Cor automated sequencer (Li-Cor BioSciences GmbH, Bad Homburg, Germany).
Molecular cytogenetic diagnosis for aneuploidies was performed on first PBs after ICSI. Infertile couples, with women aged between 31 and 41 years, gave informed consent after being counseled about the diagnosis by FISH. Polar bodies were obtained after laser assisted biopsy and fixed on slides. Telomere-specific probes for chromosomes 15, 16 and 22 (Q-Biogene, Livingston, West Lothian, UK) were used. Hybridization, post-hybridization washes and detection were according to the recommendation of the manufacturer. Fluorescence signals were detected with the Axioplan 2 microscope (Zeiss, Göttingen, Germany).
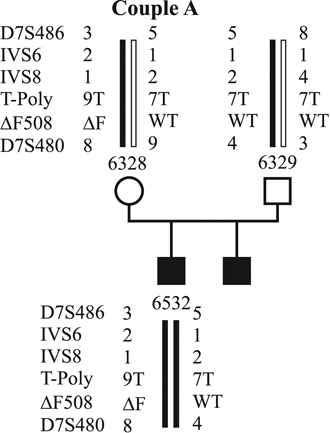
Figure 1. Haplotypes of family A. The affected alleles are marked by red bars, the wild type alleles by green bars.
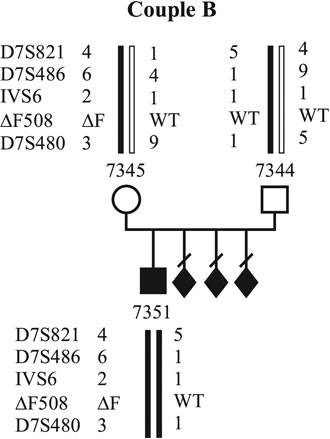
Figure 2. Haplotypes of family B. The affected alleles are marked by red bars, the wild type alleles by green bars
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

