


RNA enzymes (Ribozymes)- gene therapy applications for clinical medicine
Toudjarska I1, Kremensky IM1, Mitev VI2*
*Corresponding Author: Professor Dr. Vanio I Mitev e-mail: mitev@medfac.acad.bg
page: 3
|
|
INTRODUCTION
There are two different methods for therapy that target the genetic basis of disease - replace or eliminate. For recessive disorders, where the pathology is caused by mutation in both alleles of a gene, delivery of one normal copy will correct the phenotype. For disorders with dominant mutations (as the case with Osteogenesis imperfecta, retinitis pigmentosa etc.) or hyperactivation (in case of oncogenes and some neurodegenerative diseases), with one normal copy of the same gene present, introducing a normal gene will not work. In these cases, expression of the defective genes must be eliminated or at least limited. This is also a valid strategy in the case of viral diseases as HIV or HCV infection. For such therapy, where down regulation of a particular gene transcript is required, ribozymes are of particular interest for their ability to cleave specific mRNA.
RNA enzymes
Ribozymes are RNA molecules with catalytic activity. Since their discovery in 1982 1, RNA catalysis have been described in several essential biological processes such as RNA splicing, RNA processing, replication of RNA genomes, translation 2. Over the last two decades, numerous natural RNA motifs with catalytic activity have been identified and many more engineered through methods of in vitro evolution 3. Despite their structural diversity, RNA enzymes catalyze only a few reactions. All naturally occurring ribozymes, except the ribosomal RNA, catalyze the cleavage or ligation of RNA phosphodiester backbone 4. The ribosomal RNA catalyses the peptidil transferase reaction in peptide bond formation during protein synthesis 5. There are several criteria for classification of the ribozymes. Based on their size they can be separated into two groups - large (Group I and II introns and RNase P) and small (hammerhead, hairpin, HDV and VS), and based on their composition into all RNA enzymes (all small ribozymes, group I and II introns) and ribonucleoprotein enzymes (RNase P, spliceosome RNAs, ribosome RNA) (Table 1). While RNase P and the spliceosome RNAs show activity in the absence of protein, 23S RNA has no catalytic activity outside of the ribosomal context. Most naturally occurring ribozymes catalyze intermolecular reactions. Using mutational mapping their catalytic centers have been identified and later engineered to create trans-acting enzymes that catalyze the cleavage of other RNA molecules 6 7.
The ability to inhibit specific genes after gene transfer of antisense expression cassettes was first demonstrated by Pestka et al. in bacteria 8 and in eucaryotic cells by Izant and Wientraub 9. Following these initial studies, numerous reports describe the utilization of antisense RNA for inhibition of wide range of mammalian genes. However, these studies also indicate that the efficiency of gene inhibition was usually dependant on the presence of considerable excess of antisense RNA 10.
The discovery that ribozymes can catalyze chemical reactions with multiple turnover in-trans created great interest for their development as therapeutic agents. Although some therapies explore the possible use of RNase P and group I introns, major interest for gene therapy application are the hammerhead and hairpin ribozymes (Fig 1a). Unlike other ribonucleases, ribozymes cleave their targets in sequence specific matter, being able to distinguish single nucleotide mismatch 11.Their specificity to a particular cleavage site is determined by base complementarity between the enzyme’s binding arms and the target molecule. Different types of ribozymes have different sequence requirements for the cleavage site. For example, the hammerhead ribozyme cleaves after NHH three-nucleotide, where N can be any base and H- any except G 12. After recognizing and binding to the target sequence, the chemical cleavage proceeds rapidly (Fig 1b). The rate-limiting step of the catalytic reaction is the product release. The reaction speed can be increased if the hybridizing arms of the ribozyme are relatively short- 6 to10 nucleotides. After the product release, the ribozyme recycles and can repeat the process multiple times in vitro 6 (Fig 1b).
Ribozyme delivery
There are two basic strategies for drug delivery – direct (in vivo) delivery- through systemic or local application, and ex vivo delivery- through stable transduction of cells in vitro and their reintroduction into the patient. The successful use of the ribozymes as therapeutic agents depends on several factors. One of these factors is their stability. The half-life of RNA in serum and bodily fluids can be increased significantly by chemical modifications. Blocking the 3’ end of the molecule and different 2’ modifications of the pyrimidine bases protects from nucleases without compromising the catalytic activity 13. Phase I clinical trials demonstrate that such ribozymes are well tolerated without significant side effects 14. One other factor is the co-localization of the ribozyme and target mRNA. Several studies show enhanced intracellular activity using viral packaging signals or endogenous genes subcellular localization signals for expression of the ribozyme and target mRNA 15 16. Critical factor for ribozyme’s efficacy in vivo will be the ability to efficiently deliver the molecules into the appropriate cell types in the target organ. Numerous delivery vehicles and methods have been developed. One of the earliest approaches, based on viral vectors can deliver to different cell types with variable efficiency. Retroviral and adenovirus-5 based vectors have the disadvantages of potential generation of replication competent viruses, induction of immune response in patients, as well as random integration in the genome. Adenovirus-associated vectors do not stimulate cell-mediated immune response and show long-term transduction of infected cells 17 and are further evaluated. More recently, different liposome-based carriers and polymer-based nonviral vectors have been developed. These delivery vehicles have the advantages of lack of specific immune response, controlled release in response to environmental changes and facilitate intracellular delivery 18.
Group |
Ribozyme |
Size (nt) |
Catalytic activity (reaction product) |
|
Self-splicing introns |
Group I
Group II |
200-3000 |
Self-splicing via transesterification (3’- hydroxyl group) |
|
Small ribozymes |
Hammerhead
Hairpin
Hepatitis Delta Virus
Varkud satellite |
40
70
90
160 |
Self-splicing via transesterification (2’3’-cyclic phosphate group) |
|
Ribonucleoproteins |
RNAse P
Spliceosome
(U2 and U6 snRNAs)
Ribosome
(23S RNA) |
300
180, 100
2600 |
Pre-tRNA processing via hydrolysis (3’-hydroxyl group)
RNA splicing via transesterification (3’-hydroxyl group)
Peptidil transfer (amide) |
Table 1 Naturally occurring ribozymes and ribonucleoprotein enzymes. In Table 1 are outlined the different groups of ribozymes, the type of chemical reactions they catalyze, their size and relative abundance.
Anti-cancer ribozymes
In cancer cell, series of genetic and epigenetic events lead to unrestrained division. Molecular events that lead to transformation are two types- gain-of-function of proto-oncogenes due to mutation, gene amplification and chromosomal rearengements or loss of function due to mutations in tumor suppressor genes. Numerous teams investigate the use of ribozymes in the context of different cancers. Some targeting mutations in cancer relevant genes, some targeting genes important for cancer growth and metastasis. Zhang et al. demonstrate anti-tumorogenic effect in vivo of anti-K-ras rybozyme in mouse xenograft model for non-small cell lung cancers 19. Intratumoral injection of the adenoviral ribozyme construct resulted in complete tumor regression. Mendoza-Maldonado et al. demonstrated markedly reduction in bcr-abl mRNA levels in transduced primary hematopoetic cells from patients with chronic myelogenous leukemia 20. The ribozyme was delivered to the cytoplasm by retroviral vector. Aigner et al. show that 50% reduction in the mRNA levels of Fibroblast Growth Factor- Binding protein (FGF-BP) inhibits proliferation of prostate cancer cells in vivo 21. The first ribozymes to enter clinical trials were ANGIOZYME (AZ) and HERAZYME (HZ). AZ is stabilized ribozyme targeting flt-1 mRNA, which encodes for the receptor for vascular endothelial growth factor-1 (VEGF-1). VEGF-1 is angiogenic cytokine required for tumor neovascularization and one of the main cancer targets. Results from phase I trials show that daily intravenous and subcutaneous delivery is well tolerated and plasma levels can be maintained for prolonged periods after s.c. delivery 14. The results from phase II trials for AZ alone and in combination with chemotherapy in patients with colorectal cancer are currently being evaluated. HZ targets human epithelial growth factor receptor-2 mRNA. Phase I trial data shows no adverse effects after daily s.c. administration. Results from phase II clinical trials in patients with breast cancer are currently being evaluated. One of the problems of chemotherapy is drug resistance. One of the mechanisms of multidrug resistance (MDR) is overproduction of P-glycoprotein (P-gp). Several studies target the mRNA encoding for P-gp (MDR-1). Delivery of anti-MDR-1 hammerhead ribozyme by Kobayashi et al. using liposomes resulted in reversal of drug resistance associated with decrease in both MDR-1 expression and P-gp levels in breast cancer cell lines 22 .
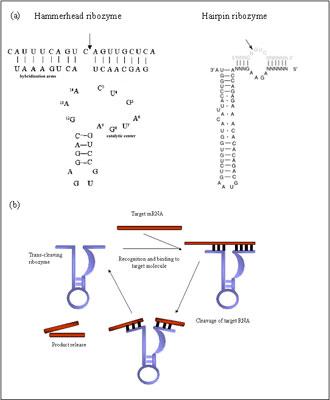
Figure 1 (a) Schematic representation of the 2D-structure of hammerhead and hairpin ribozymes. Arrows denoted the positions of the cleavage sites.
Anti-viral ribozymes
A major focus of research on ribozyme therapy has been on blocking the replication of retroviruses and RNA viruses- HIV-1, hepatitis C and hepatitis B viruses. Both hammerhead and hairpin ribozymes have been shown to protect transduced cultured cells from HIV-1 infection 23 24. Because of the lack of good animal models for these viral diseases efficacy in vivo is assessed in clinical trials. Phase I clinical trials for anti-HIV-1 ribozymes suggest that transfer of ribozyme transduced cells into the patients is well tolerated and these cells may possess transient survival advantage over control transduced cells 25. Stabilized synthetic ribozymes, targeted to HCV RNA have been designed and tested. Macejak et al. have demonstrated >98% reduction in the replication when the ribozyme was delivered with cationic lipids in combination with current treatment drug interferon 26 The same group demonstrated hepatocyte localization and retention in liver of ribozyme delivered i.v. or s.c. in mice 27. Several studies compared chemically stabilized ribozymes designed against HBV RNA. Morrissey et al. demonstrate reduced HVB DNA and antigens levels in vitro on cell culture and in vivo after delivery with implanted osmotic pump 28.
Ribozymes for genetic diseases
Ribozymes are suitable for gene therapy applications in the case of dominant disorders, where the pathology is due at least in part to a gain of function mutations (Osteogenesis imperfecta, retinitis pigmentosa, Marfan syndrome, amyotrophic lateral sclerosis etc.). Several groups demonstrate in vitro and in cellular activity of hammerhead ribozymes targeted to different mutations in COL1A1, causing Osteogenesis imperfecta 29 30. Osteogenesis imperfecta and other diseases are often genetically heterogeneous. Development of “designer” therapies for each mutation might not be practical, therefore mutation independent targeting strategies have been developed employing ribozymes recognizing and cleaving at SNP sites 31. Lewin et al. demonstrates photoreceptor rescue after treatment with hammerhead ribozyme in rat model of autosomal dominant retinitis pigmentosa 32.
Some disorders, where increase of the levels of certain protein leads to disease progression, may also benefit from ribozyme gene therapy (rheumatoid arthritis, systemic lupus erythematosus, hypercholesterolemia). Suzuki et al. use synthetically modified hammerhead ribozyme against V3-7, gene encoding for nephritogenic anti-DNA Ab in systemic lupus erythematosus. Ribozyme delivery in vivo in mouse model significantly decreases the levels of anti-DNA Ab in blood 33. Takahashi et al. targeted tumor necrosis- a (TNF-a) I- important factor in the pathogenesis of rheumatoid arthritis. Treatment with antibody to TNF-a resulted in significant improvements but there is possibility of immunological reaction to it. Delivery of chemically stabilized hammerhead ribozyme to synovial cells lacked citotoxicity and inhibited expression of TNF-a mRNA and secretion of TNF-a and IL-6 34.
Complete suppression might be difficult to achieve in some cases. In some disorders it might be necessary to block expression of the disease allele completely, for example in OI even 10% expression of the mutant allele leads to dominant-negative effect on collagen formation. Where in other diseases like atherosclerosis, where hypercholesterolemia plays key role in disease progression, any reduction in cholesterol levels will be beneficial. According to the American Hearth Association 10% reduction in cholesterol levels will prevent death from myocardial infraction in 10-25% of the population. Using viral mediated delivery of hammerhead ribozyme Enjoji et al. achieved 40% reduction in total plasma cholesterol in mouse model of atherosclerosis for 35 days after delivery 35.
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

